Hydrocarbons: formation and uses | Settala Gas
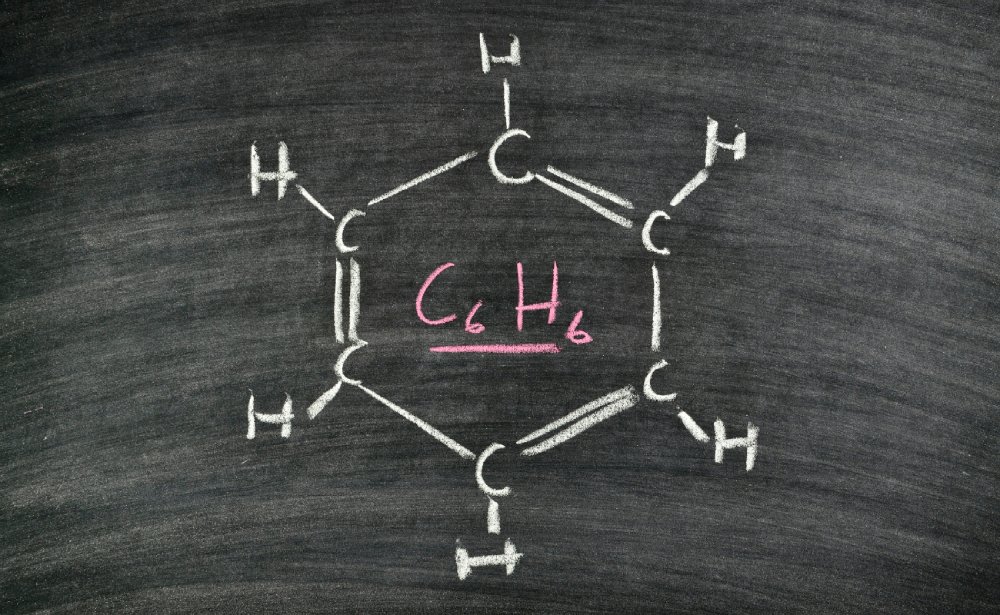
Hydrocarbons, starting from the simplest one or methane, are formed under certain conditions of temperature, pressure present in the Earth, and have an abiotic origin. Hydrocarbons are composed of carbon and hydrogen and these molecules are the main constituents of oil and natural gas. By studying the thermochemical properties of hydrocarbons, it is possible to analyse the carbon reserves present on the Earth and the cycles of formation.
The knowledge of geology has made it possible to learn how almost all hydrocarbons derive from the decomposition of the remains of living organisms buried under layers of sediments in the earth's crust, but the methane molecules unite and then contribute to the formation of larger hydrocarbons.
Main hydrocarbons - Oil and natural gas
More than 90% of natural gas is made up of methane and only a small part is present in other hydrocarbons including ethane, propane and butane. In addition to this in natural gas there are concentrations below 2-3% of H2S, He, N2 and CO2.
Natural gas reserves on Earth at the end of 2004 amounted to approximately 180,000 billion cubic meters (billion m3); by 'proven reserves’, we mean the quantities ascertained and recoverable using today's technologies and respecting current costs. Today the fear of a depletion of hydrocarbon gases is no longer so well founded, thanks to the improvement of extraction technologies and the development of more sophisticated localization methods, in fact, they have partially reduced and postponed the problem of the exhaustion of hydrocarbon sources.
Natural gas is used both as an energy source and as a raw material for the chemical industry and with its calorific value of about 50,000 kJ / kg; natural gas covers about 22.5% of the planet's total energy demand, equal to 10, 15 billion tons of oil equivalent. The use of methane as a raw material for the chemical industry includes the production of synthesis gas (CO + H2) by partial combustion with oxygen or steam reforming in which it is reacted with steam in the presence of suitable catalysts.
Oil is the second most important hydrocarbon for human activity and is made up of 80% aliphatic hydrocarbons and 20% aromatic hydrocarbons. Aliphatics are linear or branched alkanes (paraffins) and cycloalkanes (naphthenes), while aromatics have benzene as their progenitor.
Furthermore, the composition of oil varies according to the characteristics of the reservoir and the concentration of the different elements.
Hydrocarbons used for - Uses
The molecules of hydrocarbons are the basis of the highly versatile industrial organic chemistry, in particular of petrochemicals, which supplies indispensable products in many sectors (plastics, products, pharmaceuticals, agricultural products).
The most important hydrocarbons for domestic and industrial use are certainly alkanes (ethane, propane, butane, pentane, etc. ..., or coarse oil fractions called virgin naphtha and / or gas oils) and alkenes (ethylene, propylene, butene, isobutene, and butadiene) are useful for the synthesis of other compounds and for the production of plastics and rubbers.
Hydrocarbons with carbon atoms from C4 to C10 are used as automotive fuels; those from C9 to C16 as aviation and automotive fuels; finally, as fuels for diesel engines, hydrocarbons with a number of carbon atoms between C15 and C25.
In the context of the production, marketing and distribution of hydrocarbons, Settala Gas plays a fundamental role, which has always been a leader in the sector and with an ad hoc plant to manage and produce these substances. Discover the characteristics and uses of the hydrocarbons produced and sold by Settala Gas and the PURIF | AIR, PURI | FRIGOR, PURIF | HC and PURI | FOOD.